- Home
- pi ideal
- An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most. - Physics
An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most. - Physics
4.8 (167) · $ 27.99 · In stock
An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most.

Untitled, PDF, Heat

Random, PDF, Gases

ACP - Acidity and the multiphase chemistry of atmospheric aqueous particles and clouds

An ideal gas goes from state A to state B via three different processes as indicated in the P-V diagram-If {Q}_{1}, {Q}_{2}, {Q}_{3} indicate the heat absorbed by the gas along the
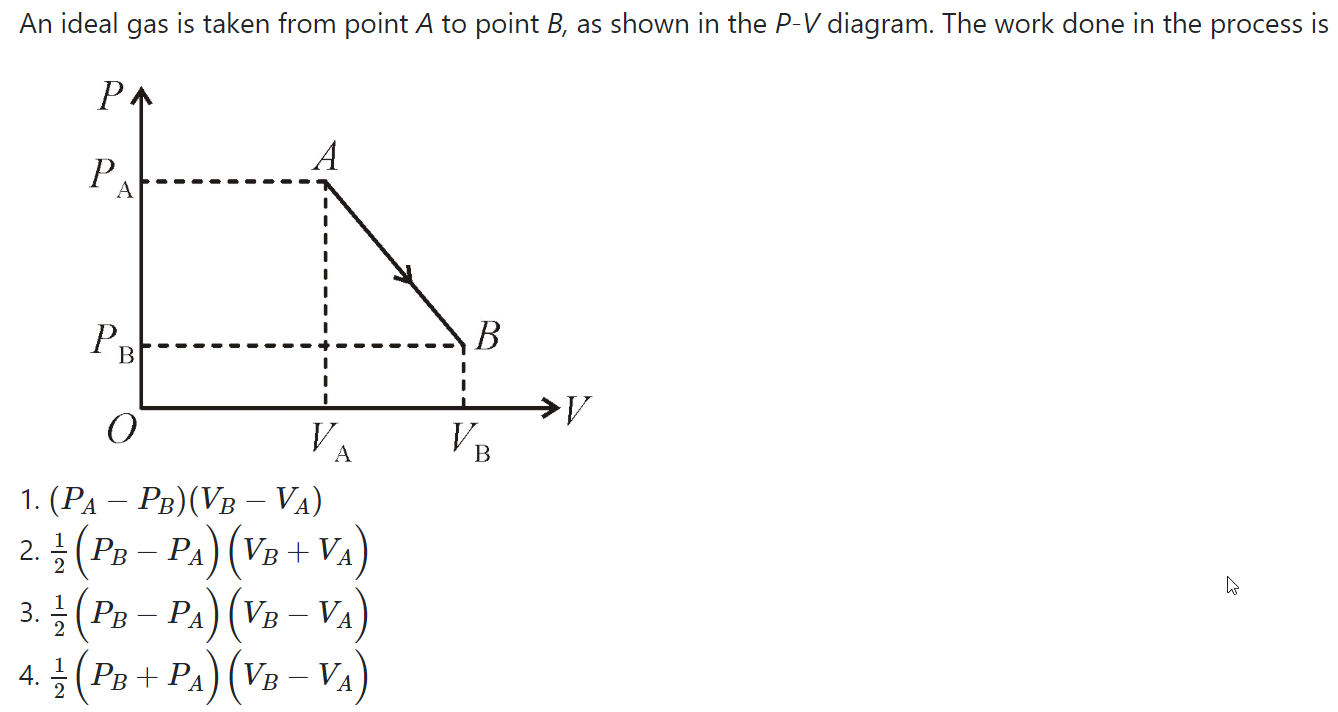
SOLVED: An ideal gas is taken from point A to point B, as shown in the P-V diagram. The work done in the process is 1. (PA-PB)(VB-VA) 2. (1)/(2)(PB-PA)(VB+VA) 3. (1)/(2)(PB-PA)(VB-VA) 4. (

An ideal gas expands from volume V_1 to V_2. This may be achieved by either of three processes: isobaric, isothermal and adiabatic. Let Delta U be the change in internal energy of

P-V diagram of an ideal gas is as shown in the given figure. Work done by the gas in the process

Ideal Gas Law Paper

Ideal Gas Law Paper

Ideal Gas Law Paper

Engineering Thermodynamics [2 ed.] 9788120338456
What are all the applications of an isothermal process? - Quora












