- Home
- tafel slope
- Calculation of the Tafel slope and reaction order of the oxygen evolution reaction between pH 12 and pH 14 for the adsorbate mechanism, Catalysis, ChemRxiv
Calculation of the Tafel slope and reaction order of the oxygen evolution reaction between pH 12 and pH 14 for the adsorbate mechanism, Catalysis, ChemRxiv
4.5 (543) · $ 29.99 · In stock
Despite numerous experimental and theoretical studies devoted to the oxygen evolution reaction, the mechanism of the OER on transition metal oxides remains controversial. This is in part owed to the ambiguity of electrochemical parameters of the mechanism such as the Tafel slope and reaction orders. We took the most commonly assumed adsorbate mechanism and calculated the Tafel slopes and reaction orders with respect to pH based on microkinetic analysis. We demonstrate that number of possible Tafel slopes strongly depends on a number of preceding steps and surface coverage. Furthermore, the Tafel slope becomes pH dependent when the coverage of intermediates changes with pH. These insights complicate the identification of a rate-limiting step by a single Tafel slope at a single pH. Yet, simulations of reaction orders complementary to Tafel slopes can solve some ambiguities to distinguish between possible rate-limiting steps. The most insightful information can be obtained from the low overpotential region of the Tafel plot. The simulations in this work provide clear guidelines to experimentalists for the identification of the limiting steps in the adsorbate mechanism using the observed values of the Tafel slope and reaction order in pH-dependent studies.

Comparative Electrocatalytic Oxygen Evolution Reaction Studies of Spinel NiFe2O4 and Its Nanocarbon Hybrids

Potential-dependent OER performance on dual-Fe-Ir sites by grand canonical based constant charge method - ScienceDirect

Quasi in situ NAP-XPS, ex-situ XAFS, and operando DEMS measurements

DFT calculation of the OER reaction mechanism to elucidate intrinsic
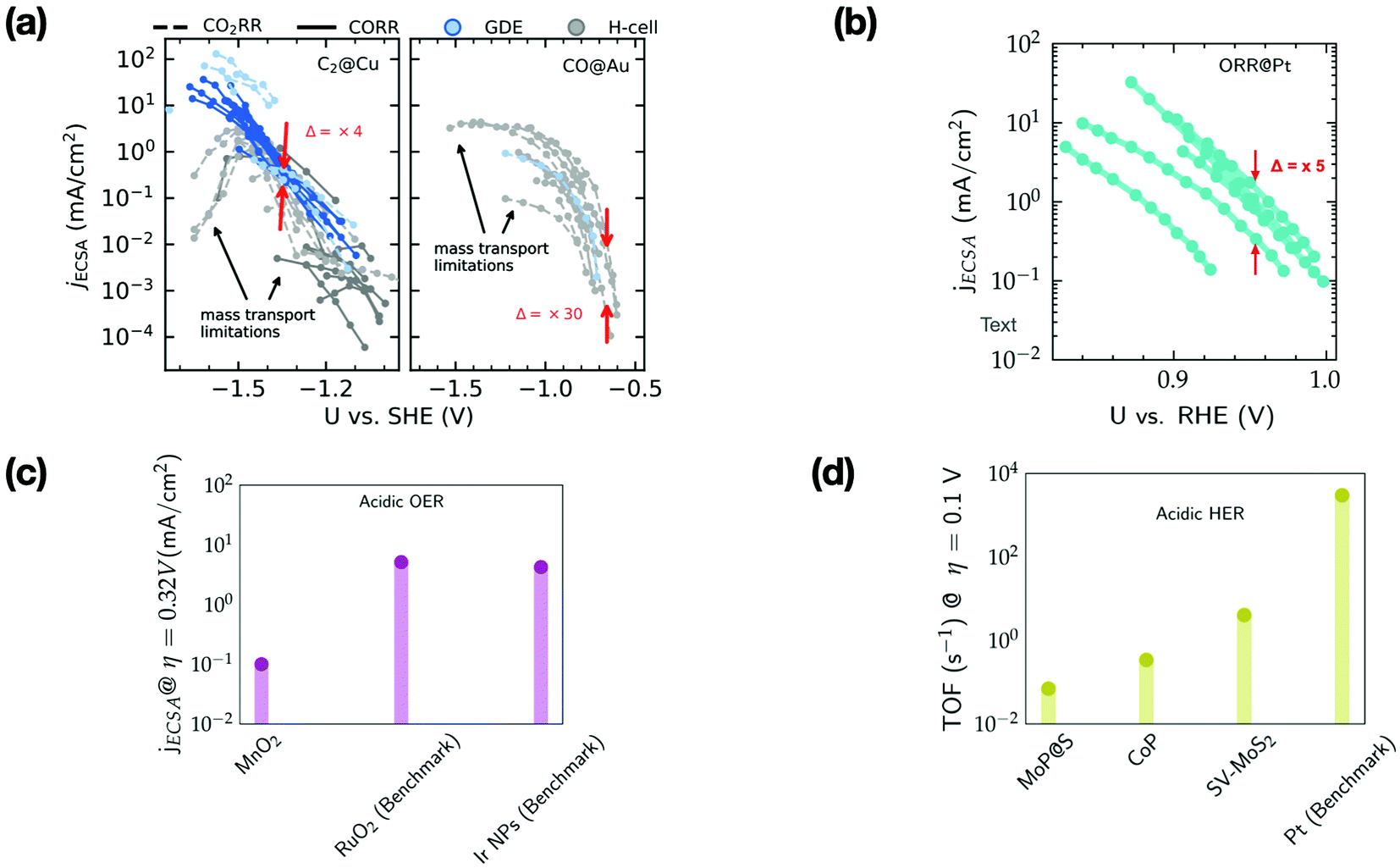
Key role of chemistry versus bias in electrocatalytic oxygen evolution
Improving the intrinsic activity of electrocatalysts for sustainable energy conversion: where are we and where can we go? - Chemical Science (RSC Publishing) DOI:10.1039/D1SC04775B
Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C6CS00328A

Modulation to favorable surface adsorption energy for oxygen evolution reaction intermediates over carbon-tunable alloys towards sustainable hydrogen production

PDF) What X-ray absorption spectroscopy can tell us about the active state of earth-abundant electrocatalysts for the oxygen evolution reaction

The rate-determining term of electrocatalytic reactions with first-order kinetics - ScienceDirect

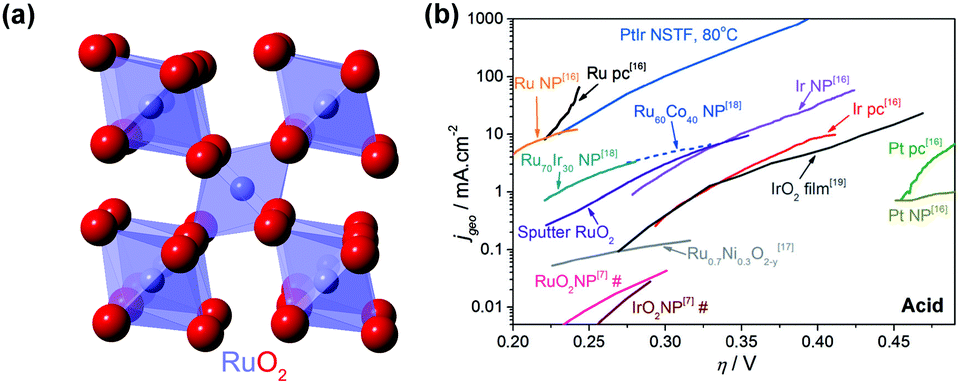
PDF) Highly efficient water oxidation via a bimolecular reaction mechanism on rutile structured mixed-metal oxyfluorides

Potential-dependent OER performance on dual-Fe-Ir sites by grand canonical based constant charge method - ScienceDirect

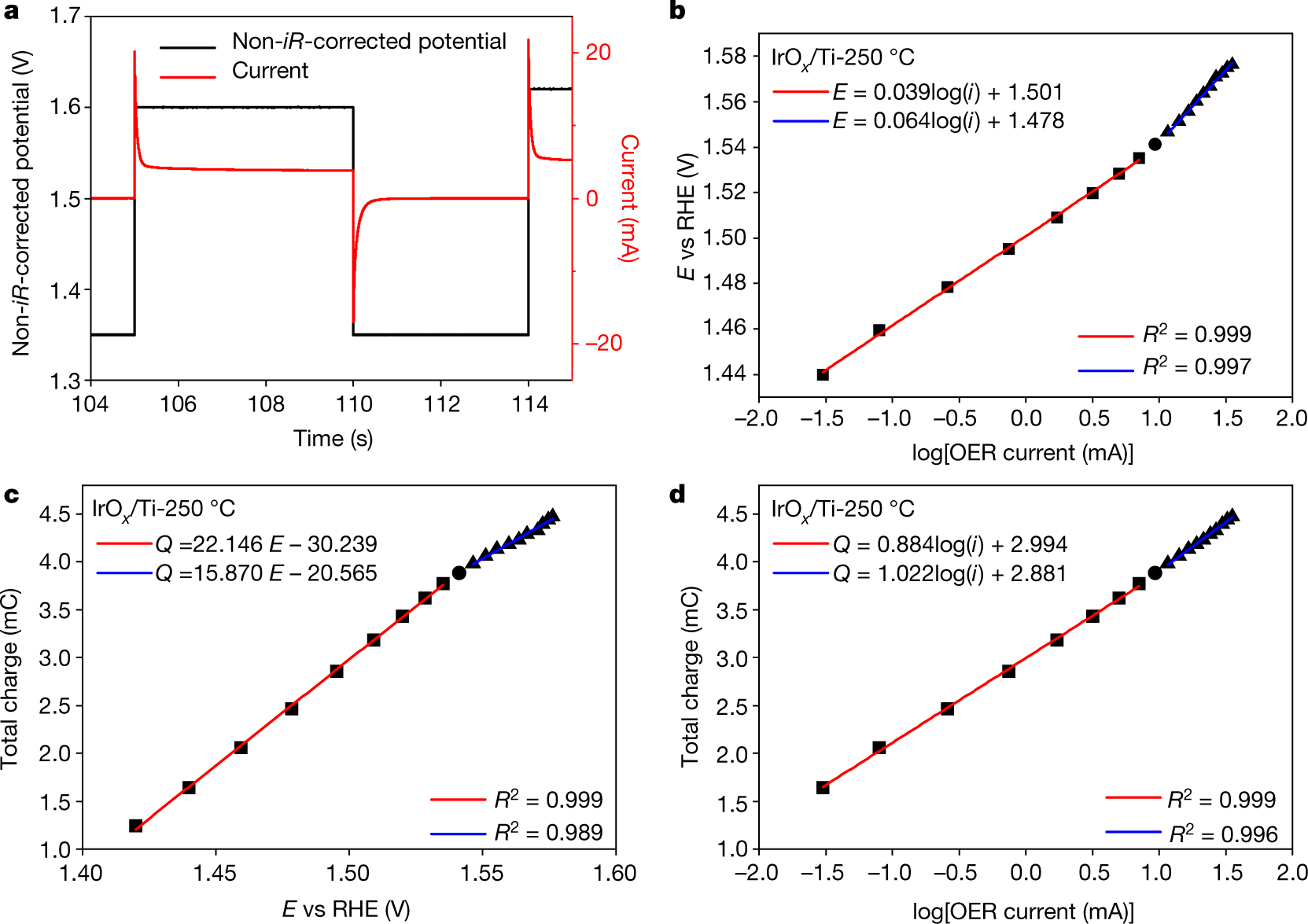
Tafel slopes and kinetic order of the OER at various pH levels over IrO