Five Common Mistakes Submitting a Premarket Notification
4.5 (277) · $ 17.99 · In stock
How you can avoid the most common errors made when submitting a 510(k), the “premarket notification,” with simple measures

FDA PMA submission process: a beginner's guide

Premarket Notification The 510(k) Process

Inclusion of PRO in summary documents, separated by endpoint
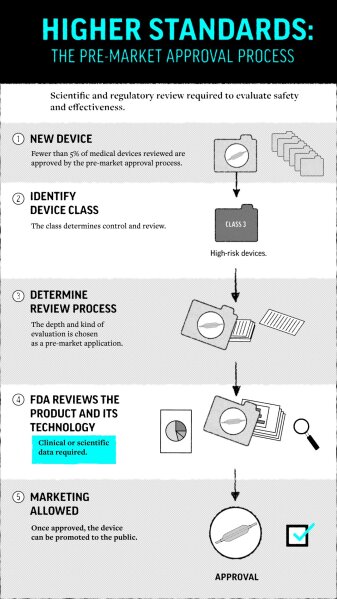
At FDA, a new goal, then a push for speedy device reviews
Cancel, Deactivate, or Reactivate a Facility Registration

FDA 510(k) Submission: A Step-By-Step Guide On How To Prepare Yours

Ketryx on LinkedIn: The latest (June 2023) changes to the FDA's new premarket submission…

FDA Releases New Cybersecurity Premarket Guidance

Boeing CEO's Comeback Plan for 2024 Takes a Blow Five Days In - BNN Bloomberg

How To Make The Most Of Your Pre-Submission Interactions With FDA

7 Common 510(k) Mistakes and How to Avoid Them

Premarket Notification The 510(k) Process
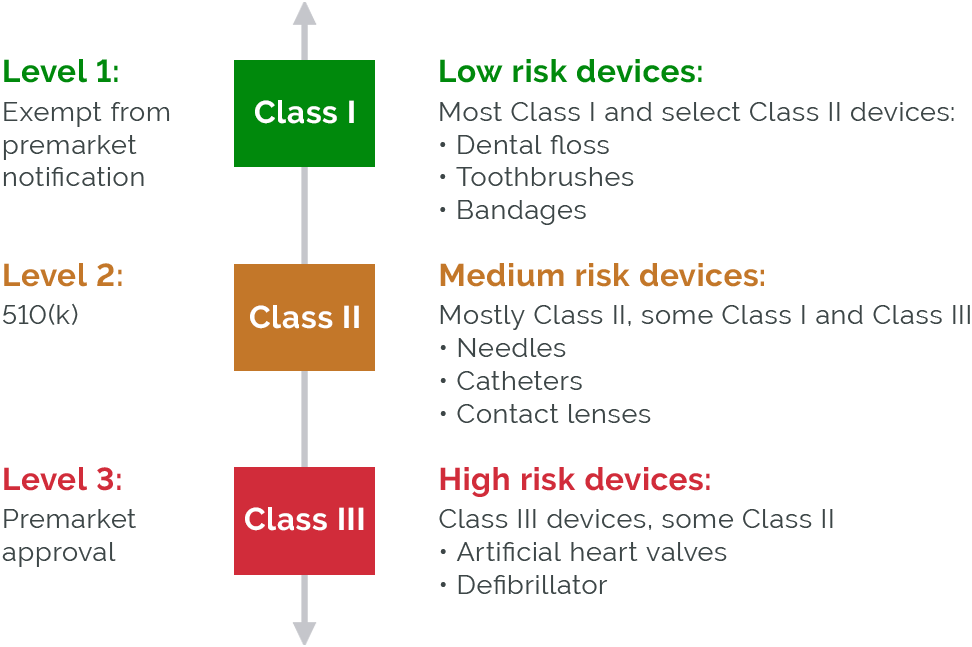
Premarket Approval (PMA) Definition

The De Novo Classification Process A Work in Progress

FDA 510(k) Submission: A Step-By-Step Guide On How To Prepare Yours












