- Home
- compressibility factor z
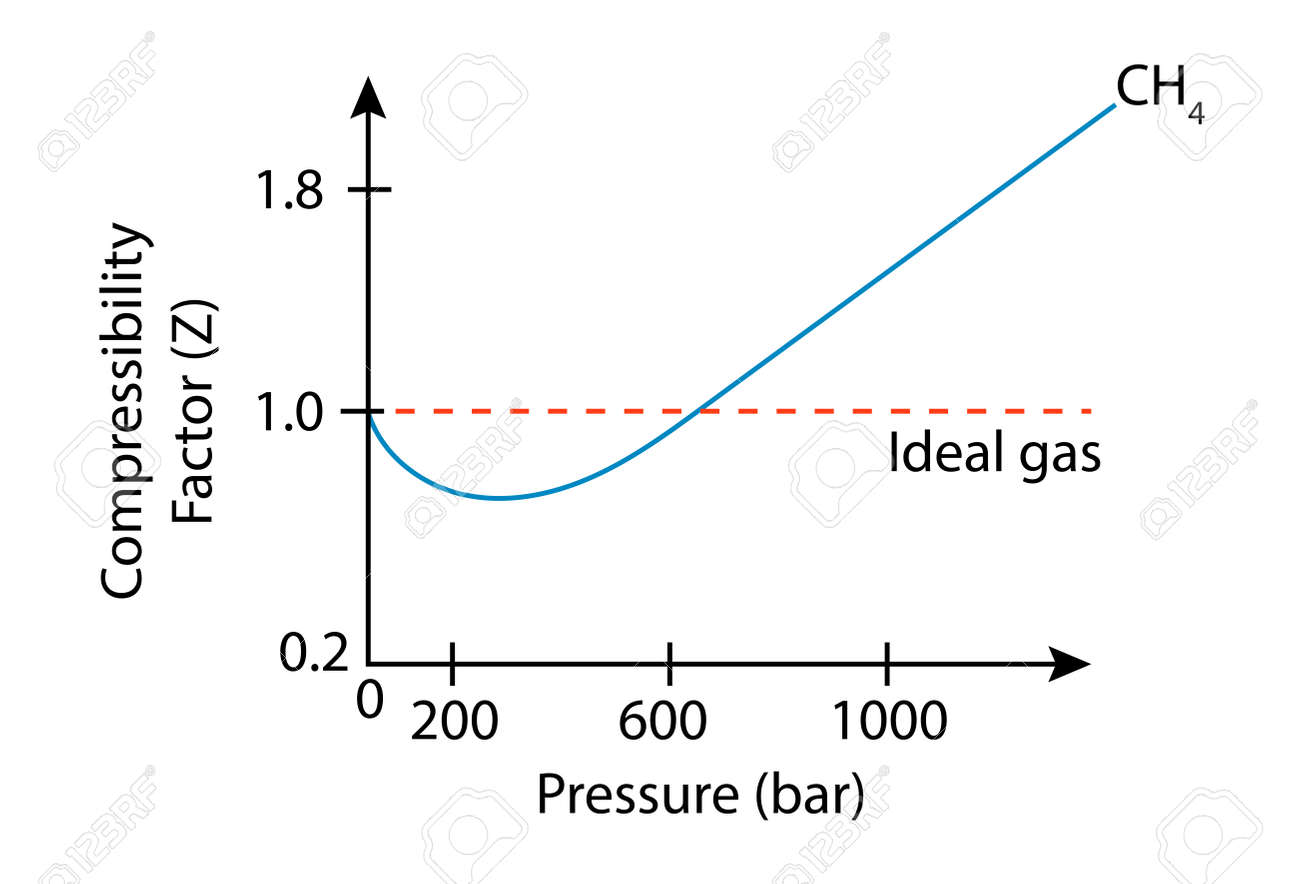
- In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar
In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar
4.8 (730) · $ 6.00 · In stock
Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases

Non-Ideal Gas Behavior Chemistry: Atoms First
Energies, Free Full-Text
Membranes, Free Full-Text

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
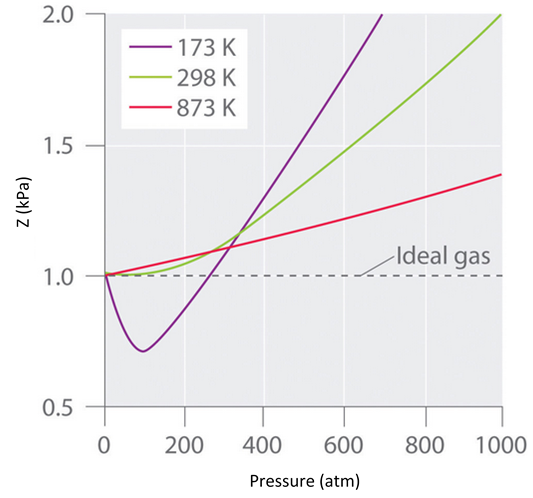
4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts
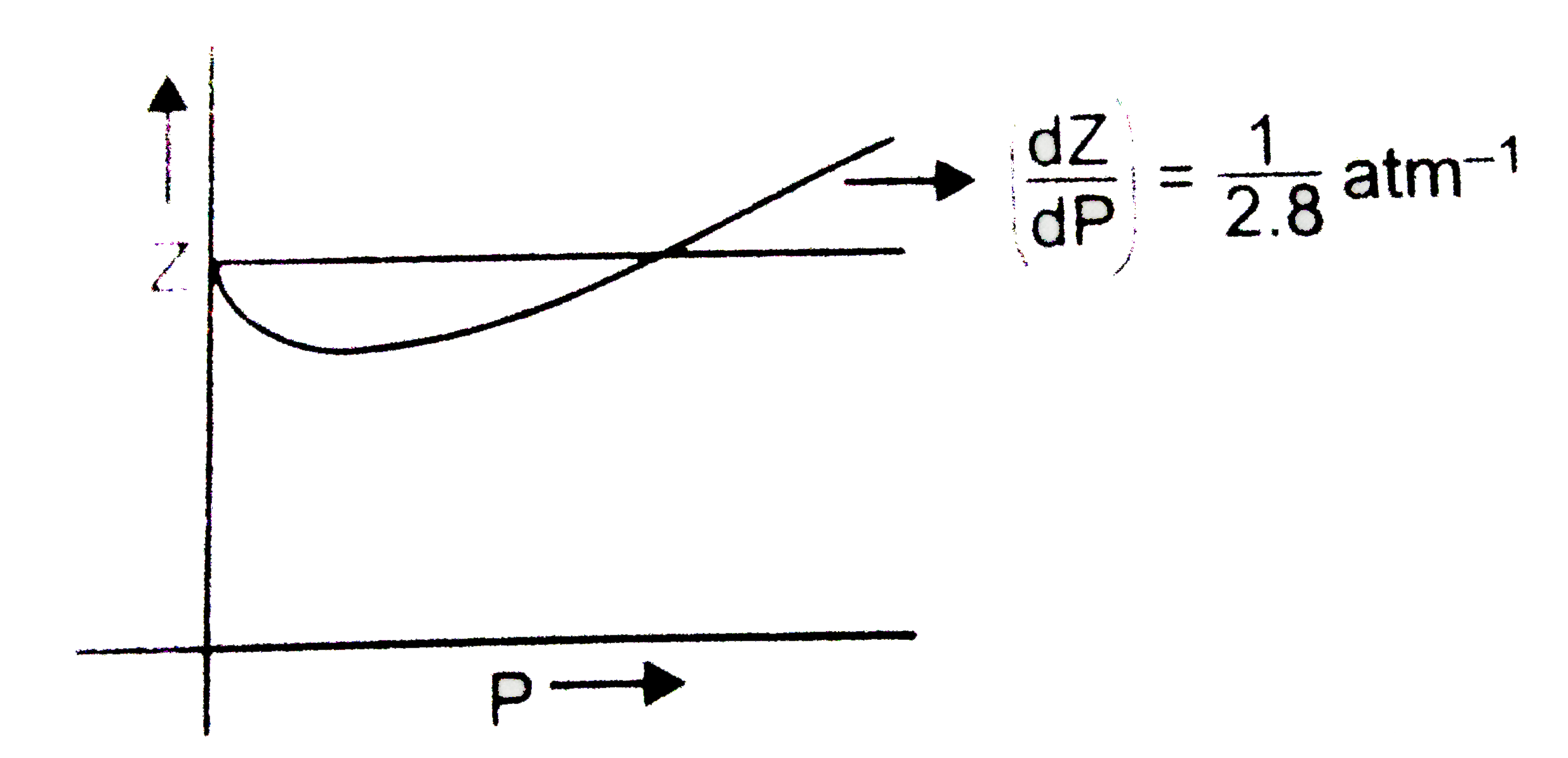
The graph of compressibility factor (Z) vs. P for one mole of a real g

WPILARIVIANN ZU 60. ollowing compressibility factor (2) vs pressure graph 300 K, the compresability of Cheatre 200 bar deviates from ideal behaviour because Compressibility Factor (2) Ideal gas 02 0 200 600

The graph of compressibility factor (Z) :vs: P one mole of a real gas is shown in following diagram. The graph is plotted constant temperature 273 K. If the slope of graph

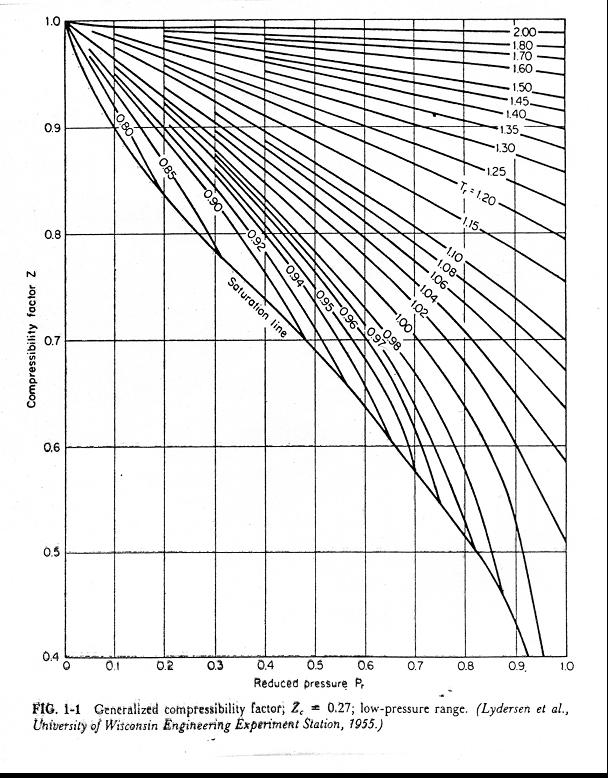
Reading Compressibility Factor Charts

Compressibility Factor Z Important Concepts and Tips for JEE Main

Sheet - 01 - Real Gas, PDF, Gases