Preparation of Standard Solution of Sodium Carbonate - Chemistry
5 (434) · $ 22.00 · In stock
A common primary standard for standardizing strong acids is sodium carbonate (Na2CO3).For acid-base titration, it is customary to prepare solutions of an acid and base of the desired concentration. Visit BYJU
A common primary standard for standardizing strong acids is sodium carbonate (Na2CO3).For acid-base titration, it is customary to prepare solutions of an acid and base of the desired concentration. Visit BYJU'S to understand more about it.
How to find the amount of Na2CO3 required to prepare 100 ml of 0.5

Standard solution, Resource

CHM231 - good - CHM 231 : ANALYTICAL OF CHEMISTRY LAB REPORT

PDF) EXPERIMENT 1 STANDARDIZATION OF HCl SOLUTION WITH Na2CO3

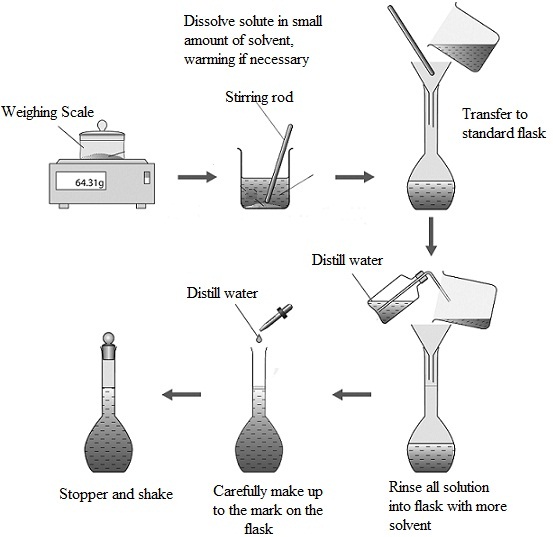
Preparing a standard solution
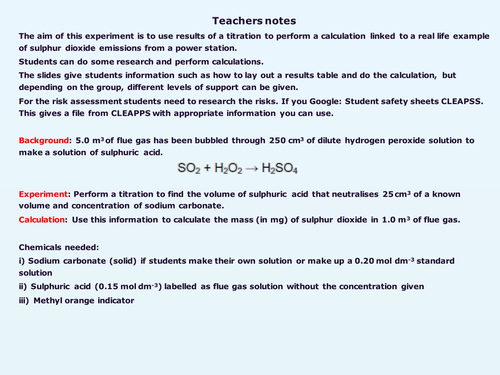
Titration investigation-Power station emissions
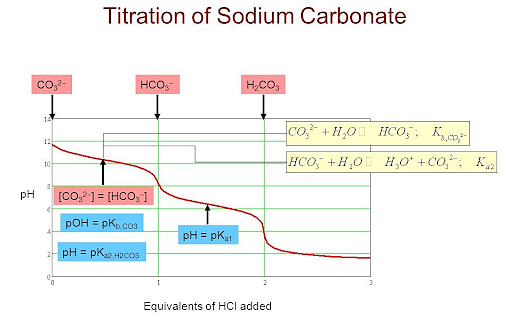
Titration of Hydrochloric Acid against Standard Sodium Carbonate
Preparation of Standard Solution of Sodium Carbonate: Theory
Acids-Bases and Salts-Volumetric analysis, Chemistry tutorial












