The FDA's rule change requiring providers to inform women about
4.7 (471) · $ 26.50 · In stock
The FDA Needs More Power to Regulate Toxic Chemicals in Cosmetics

Women Can Wait Longer Between Pap Tests, Doctor Reveal
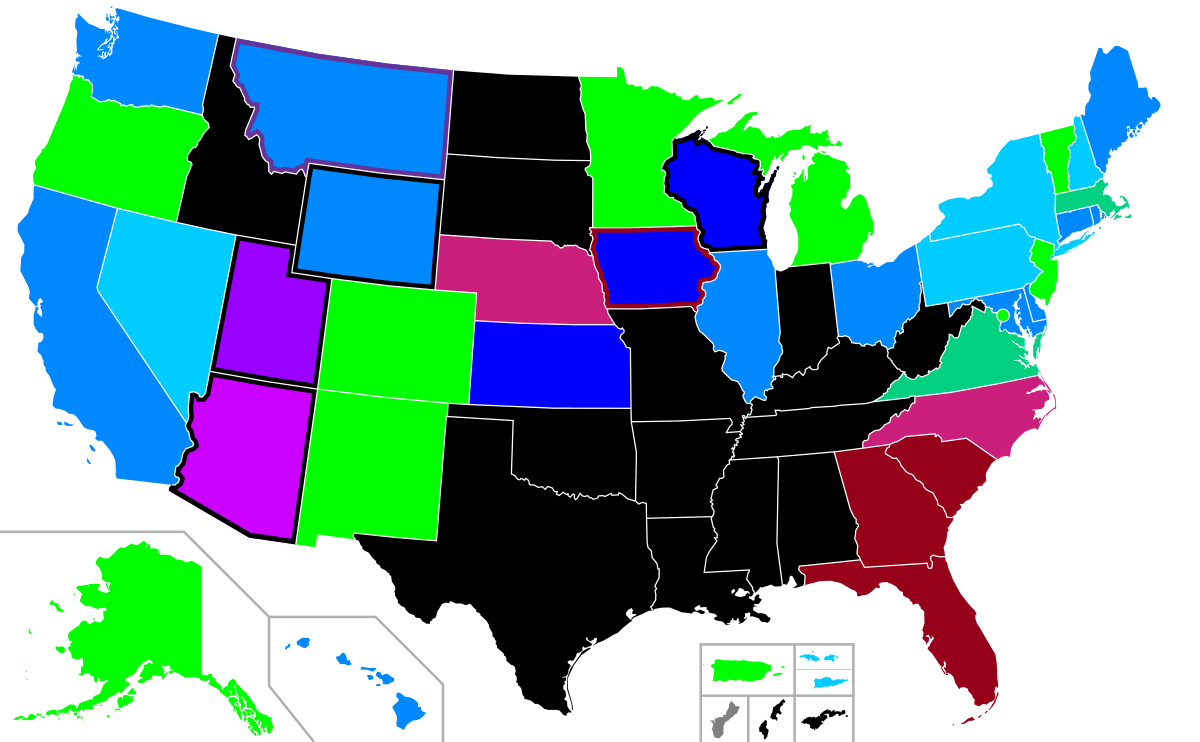
Abortion in the United States - Wikipedia

FDA Wants To Require Mammogram Providers To Notify Women About Their Breast Tissue Density - CBS New York

FDA to require mammogram providers to notify women about breast density to help detect breast cancer sooner
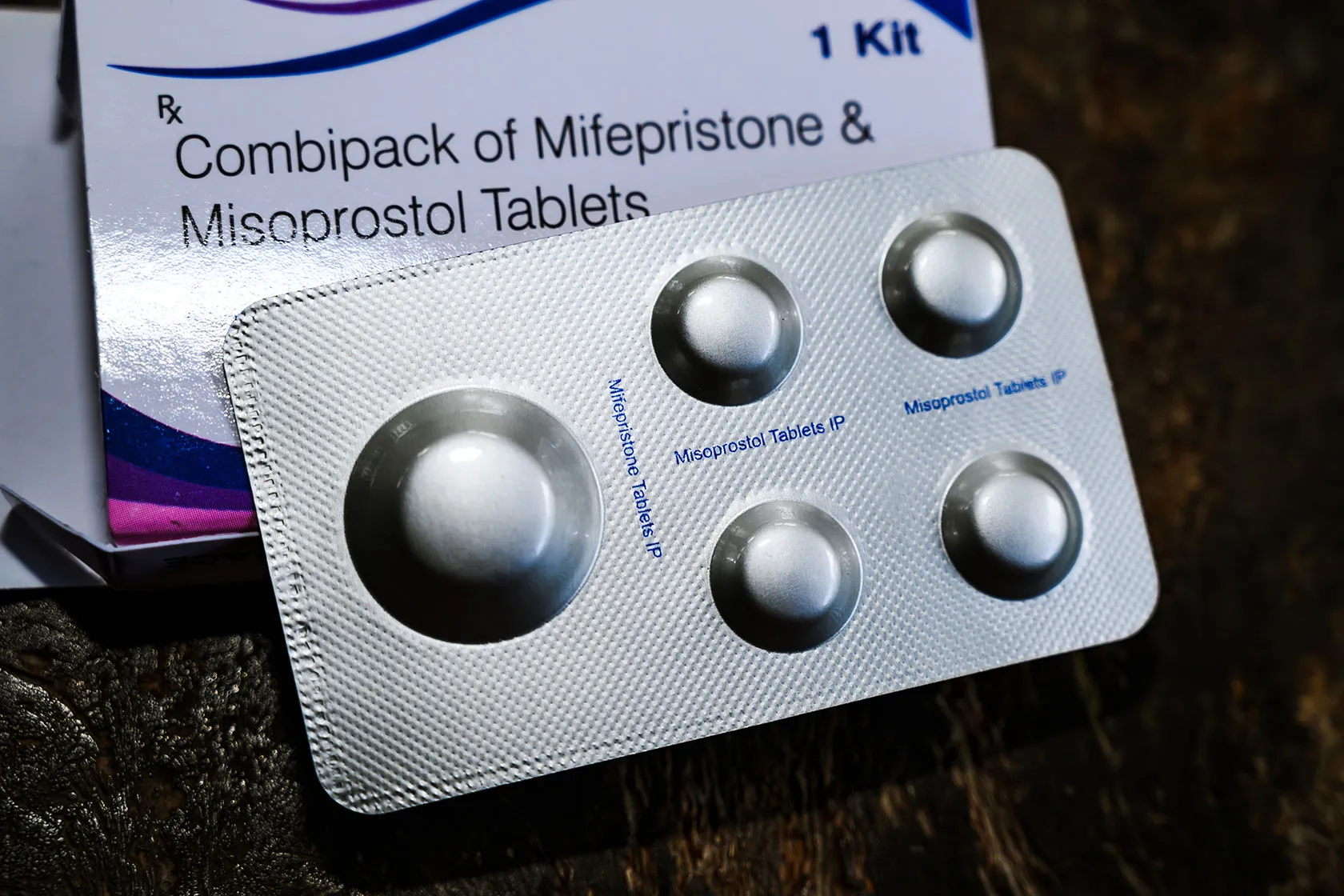
The FDA's Decisions on Mifepristone Have Advanced the Safety of Medication Abortion - Center for American Progress

Adrian Waller on LinkedIn: #finditearly #breastcancer
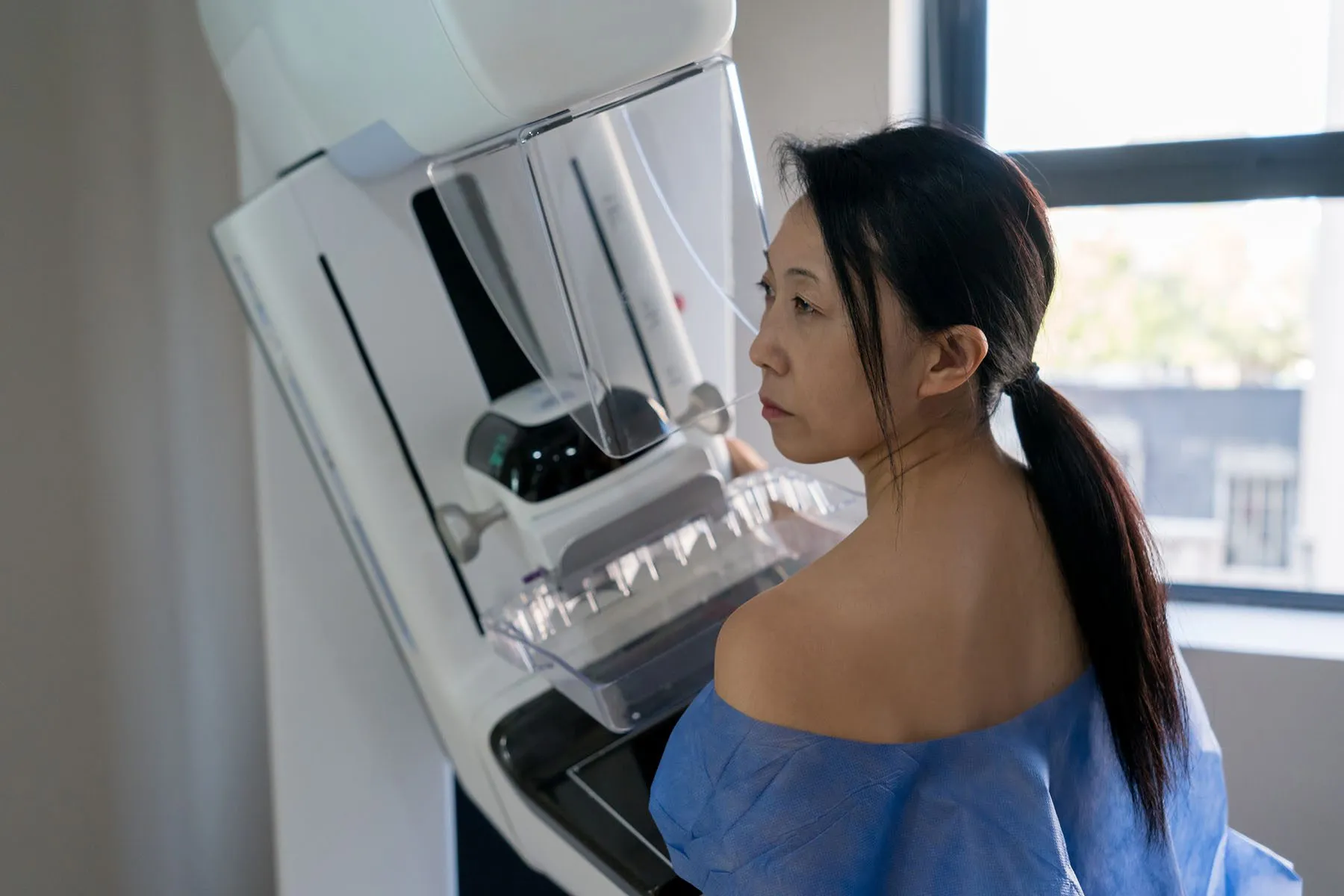
FDA will require mammogram providers to share breast density reports with patients

Changes Ahead: Abortion Policy Proposals Affecting Reproductive Medicine, American Society for Reproductive Medicine

Breast Cancer – ActiveBeat – Your Daily Dose of Health Headlines

Women's Healthcare, A Clinical Journal for NPs on LinkedIn: The

Opill, the first over-the-counter birth control pill, gets FDA approval

The medical research gender gap: how excluding women from clinical trials is hurting our health, Health & wellbeing