- Home
- compressibility factor equation
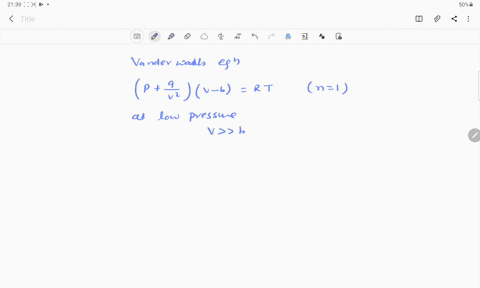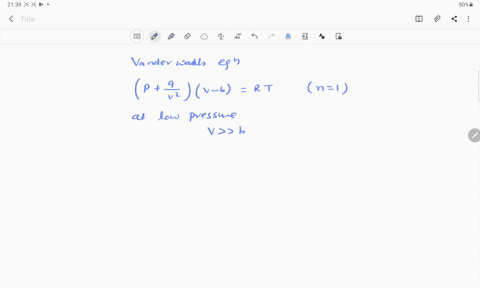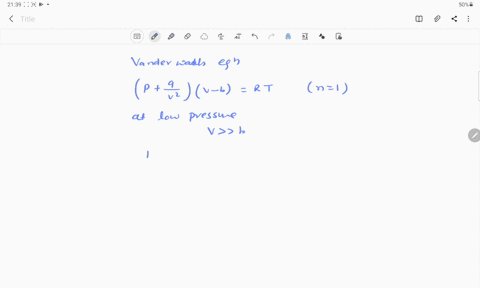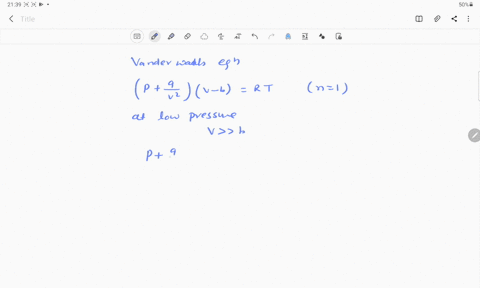
- 20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
4.5 (593) · $ 22.50 · In stock
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

At low pressures For 1 mole, the van der Waals equation is written as [ p + a / V 2] V = RT The compressibility factor is then equal to:A. 1
⏩SOLVED:At low pressures, van der Waals' equation is written as…

012 IfZ is a compressibility factor, van der Waals equation low pressure can be written as: [2014] RT I-끔 (C) Z-I+ Z=1+ (B) Ζ=I.RT (D) Z=l- _ pb VRT

⏩SOLVED:For a van der Waals gas with given values of a and b,…

Solved Problem 1: Molar Volume and Compressibility Factor

a) Compressibility factor Z obtained from the Lee-Kesler EoS, and

Bengali] What will the value of compressibility factor (Z) be for a g

Real Gases and Compressibility Factor

Solved APPENDIX Problem 1: Molar Volume and Compressibility
Van der Waals equation - Wikipedia

If Z is a compressibility factor, van der Waals' equation at low pressure can be written as

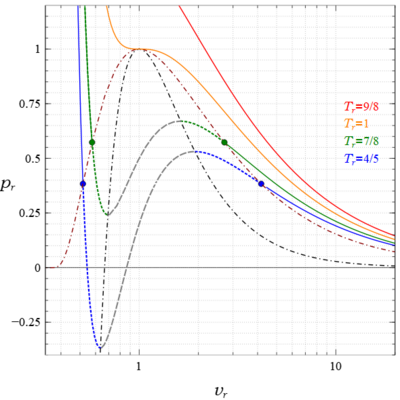
Cubic equations of state - Wikipedia

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0









