- Home
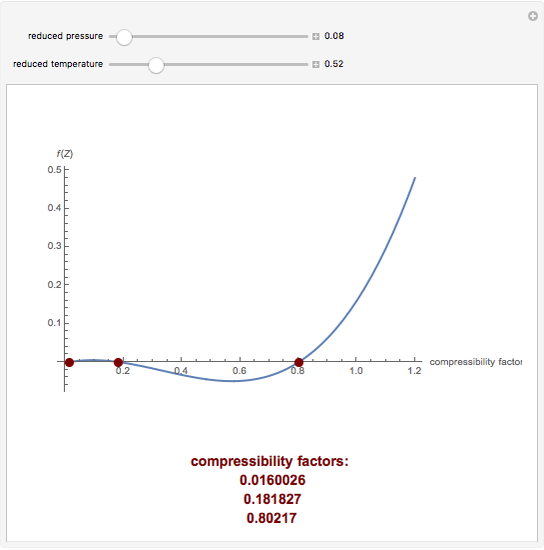
- compressibility factor equation
- Compressibility factor (Z) for a van der Waals real gas at critical point is
Compressibility factor (Z) for a van der Waals real gas at critical point is
4.7 (535) · $ 13.99 · In stock
Share your videos with friends, family and the world

At point A, which of the following is true?P: Compressibility factor becomes 0.375Q: Gas is called van der Waals gasR: Below A only one variable is required liquefactiononly Q, Ronly P, QP

Bengali] What will the value of compressibility factor (Z) be for a g
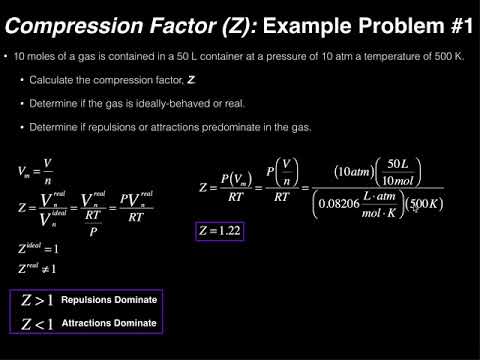
Physical Chemistry The Compression Factor (Z) [w/1 example]

The van der Waals equation of state at the critical point

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
Why there is different between the value of compressibility factor at critical point between real and ideal gas? - Quora
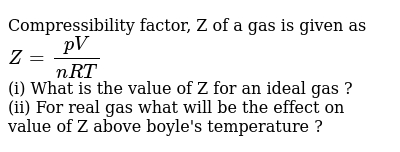
Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

Fluids, Free Full-Text
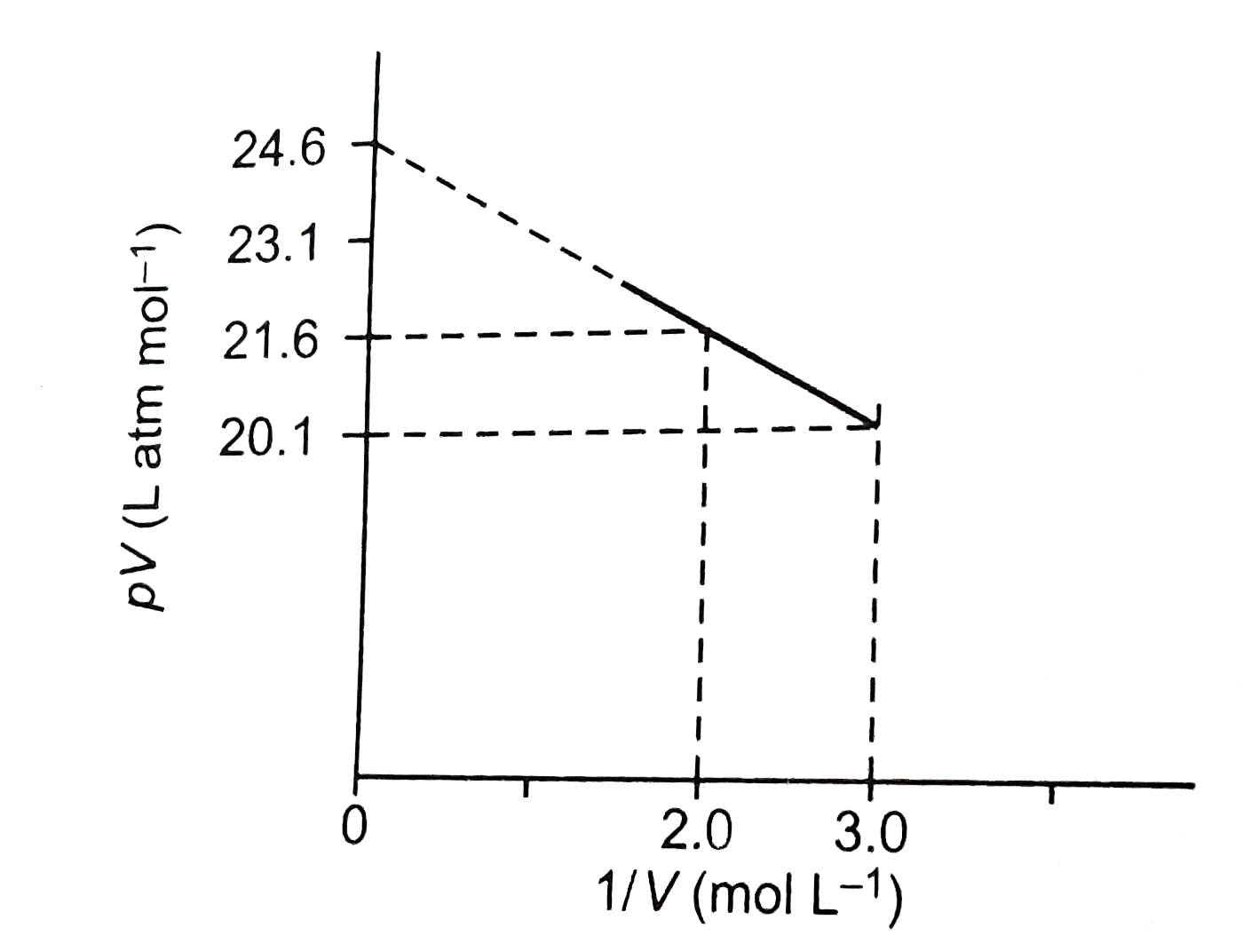
COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12

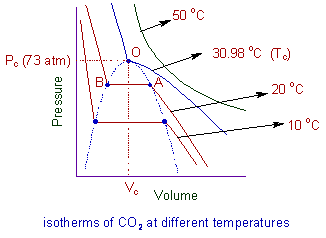
The critical pressure P(C) and critical temperature T(C) for a gas obe