- Home
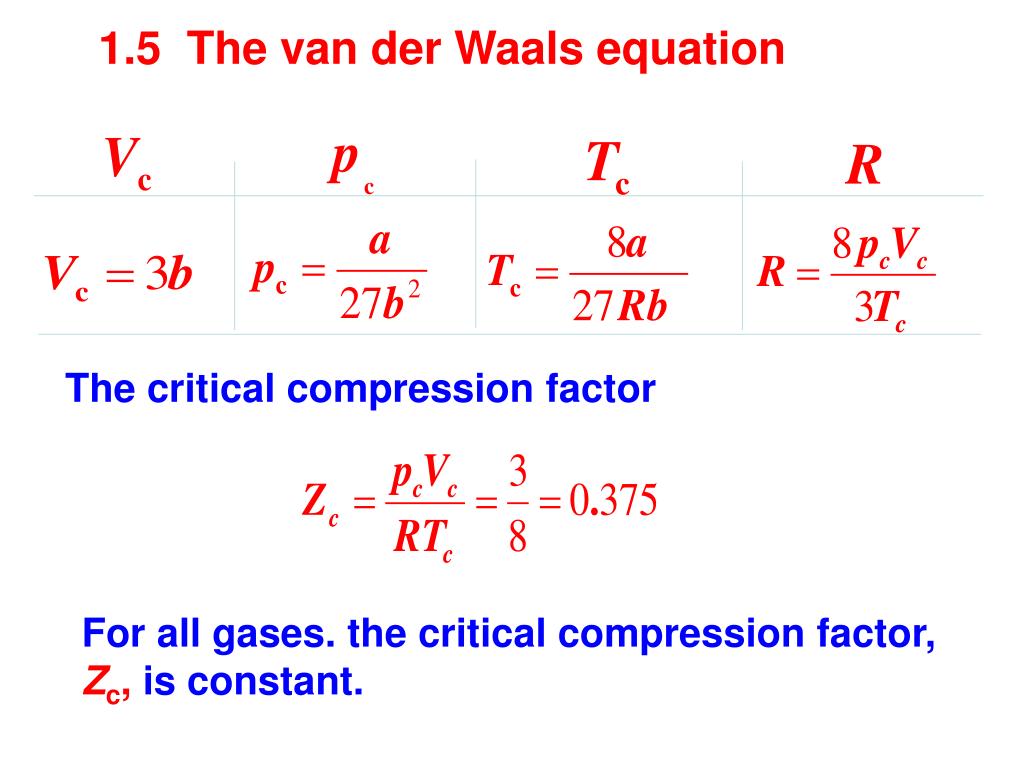
- compression factor equation
- UNUB At Boyle temperature, the value of compressi factor Z has a value of one over a wide range of pressure. This is due to the fact that in the van der
UNUB At Boyle temperature, the value of compressi factor Z has a value of one over a wide range of pressure. This is due to the fact that in the van der
4.7 (764) · $ 19.99 · In stock
Click here:point_up_2:to get an answer to your question :writing_hand:unubat boyle temperature the value of compressifactor z has a value of one over a
Click here👆to get an answer to your question ✍️ UNUB At Boyle temperature- the value of compressi factor Z has a value of one over a wide range of pressure- This is due to the fact that in the van der Waals equation -1- The constant a is negligible and not b -2- The constant b is negligible and not a -3- Both the constant a and b are negligible -4- Attraction balances repulsion

qph.cf2.quoracdn.net/main-thumb-30453142-200-bmcwo

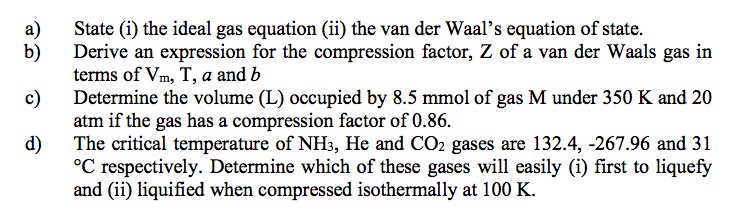
Compressibility factor, Z of a gas is given as Z = pV / nRTi What

PDF) Transport Properties of Fluids Their Correlation Prediction

At critical set of condition the value of compressibility factor

PDF) AS VT Visual Touch 2.22.2024Alternative Sight Vision

7. At Boyle's temperature, the value of compressibility factor Z

Respostas - Físico-Química (Vol.1) - Atkins PDF

vocab.txt · pile-of-law/legalbert-large-1.7M-2 at
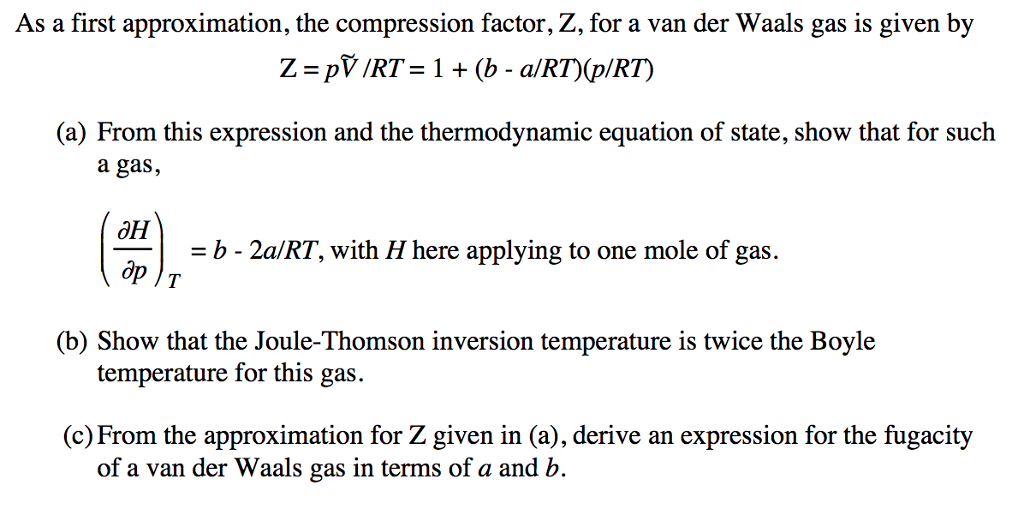
Solved As a first approximation, the compression factor, Z

Below the boyle temperature ,explain the effect of temperature on

economy Archives - Brazilian-American Chamber of Commerce



SOLVED: At Boyle's temperature, the value of compressibility

A LEVEL Heat and Modern 2016, PDF, Thermometer