Solved What is the equilibrium constant (Kp) at 45 °C for
4.6 (145) · $ 21.50 · In stock
Answer to Solved What is the equilibrium constant (Kp) at 45 °C for
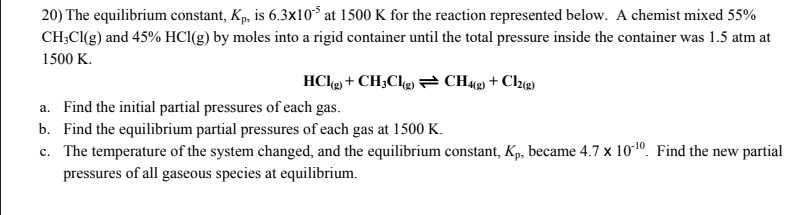
Solved 14) Will decreasing the temperature of the following

For reactions in the gas phase, an equilibrium constant may be written in terms of molarity (Kc) or in

DIU I CULILLU. The equilibrium constant Kp (in atm) the reaction is 9 7 atm and 300 K. A, (g) =B2(g) + C2 (g) Calculate the average molar mass (in gm/mol) of
How to Calculate the Equilibrium Constant, K

How to Calculate an Equilibrium Constant Kp Using Partial Pressures, Chemistry

Answered: A reaction vessel initially contains…
✓ Solved: At 2200^∘ C, Kp=0.050for the reaction N2(g)+O2(g) ⇌ 2 NO(g) What is the partial pressure of
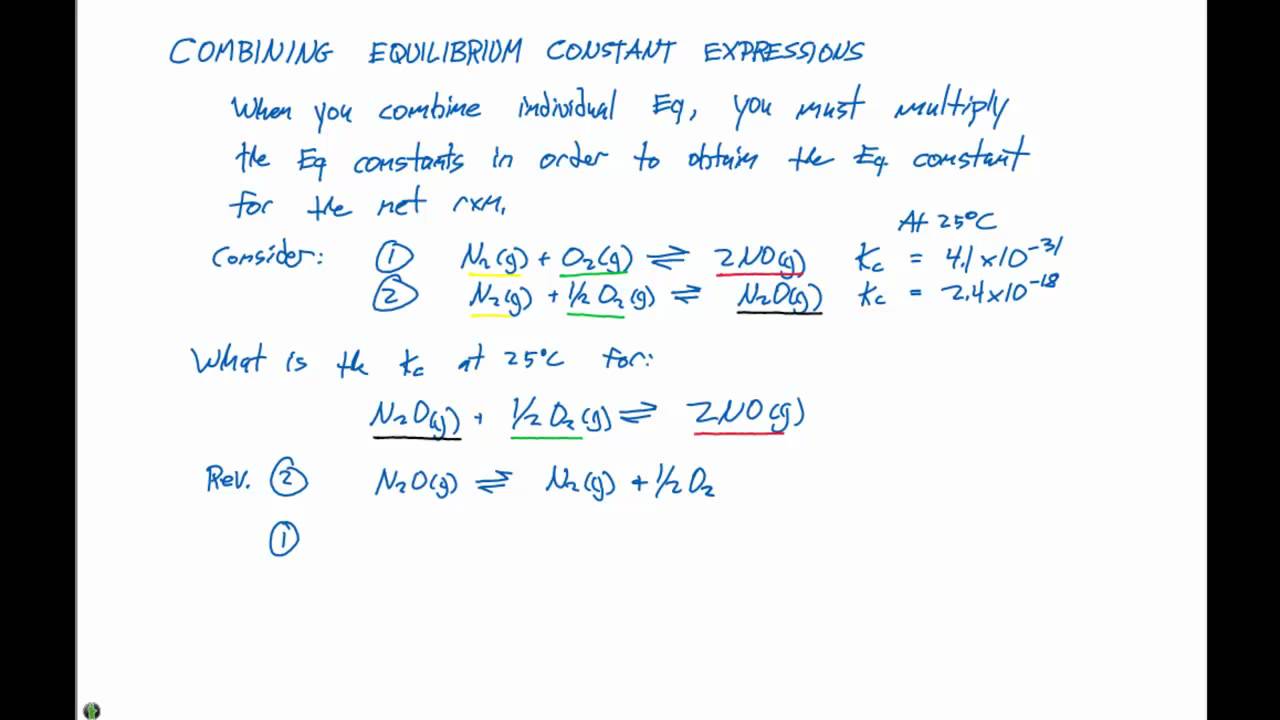
15.3 Combining Equilibrium Constants

Solved What is the equilibrium constant (Kp) at 45 °C for

Chapter 14