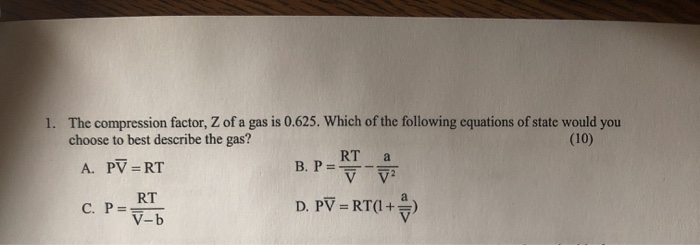At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{
4.5 (364) · $ 26.00 · In stock

Measuring Gas Pressure - Chemistry
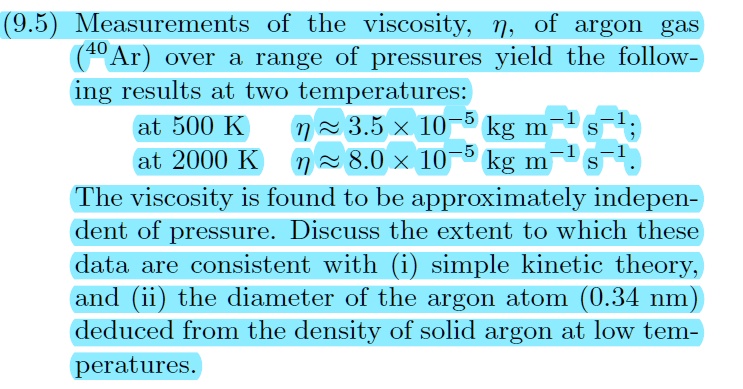
SOLVED: (9.5 Measurements of the viscosity; n, of argon gas 40 Ar) over a range of pressures yield the follow- ing results at two temperatures: at 500 K 3.5 X 10-5 kg
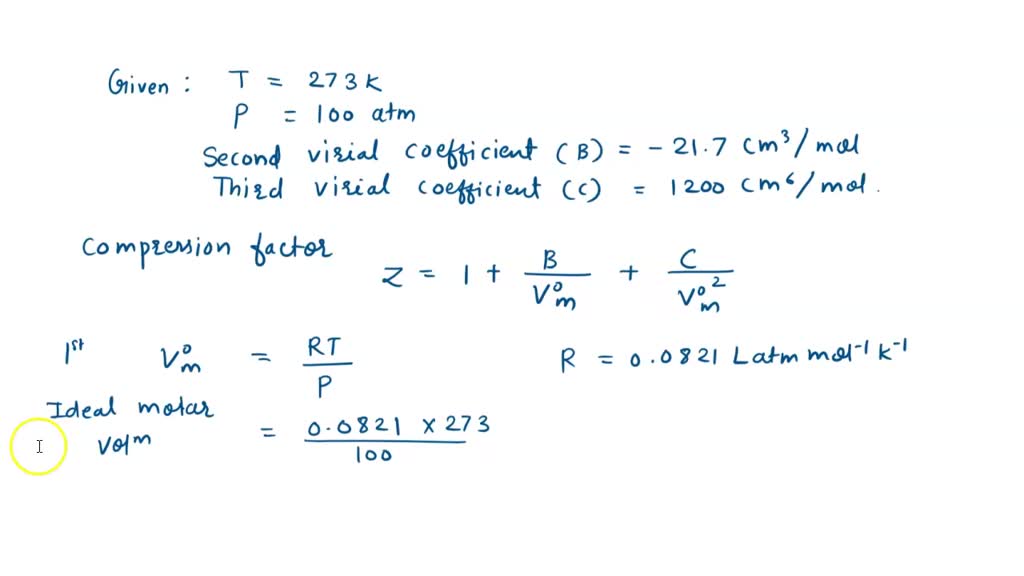
SOLVED: At 273 K, measurements on argon gave B = -21.7 cm^3/mol and C = 1200 cm^6/mol^2, where B and C are the second and third virial coefficients in the expression of

Pyrolysis of sulfonic acid substituted benzenes and investigation of CO2 capture capability of resulting carbons - ScienceDirect

The apparatus shown consists of three bulbs connected by stopcock

⏩SOLVED:At 273 K measurements on argon gave B=-21.7 cm^3 mol^-1 and…

Answered: 17) with air at a pressure of 1.00 atm.…
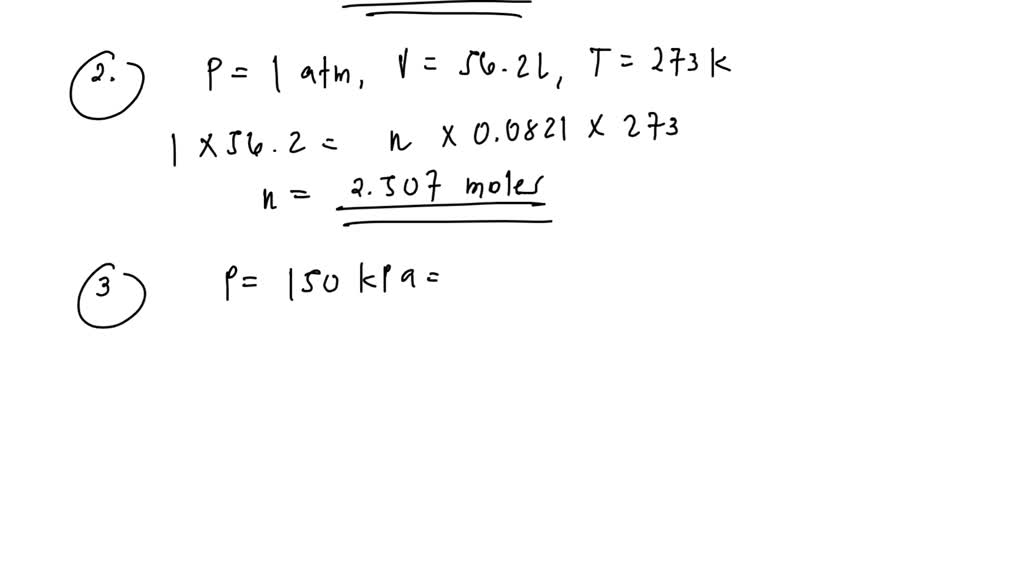
SOLVED: At what temperature will 0.654 moles of neon gas occupy 12.30 L at 1.95 atm? A sample of argon gas at STP occupies 56.2 L. Determine the number of moles of

8.2: Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law