- Home
- pressure compression
- Why do pressure and temperature increase during the compression of a gas? - tec-science
Why do pressure and temperature increase during the compression of a gas? - tec-science
4.9 (506) · $ 18.50 · In stock
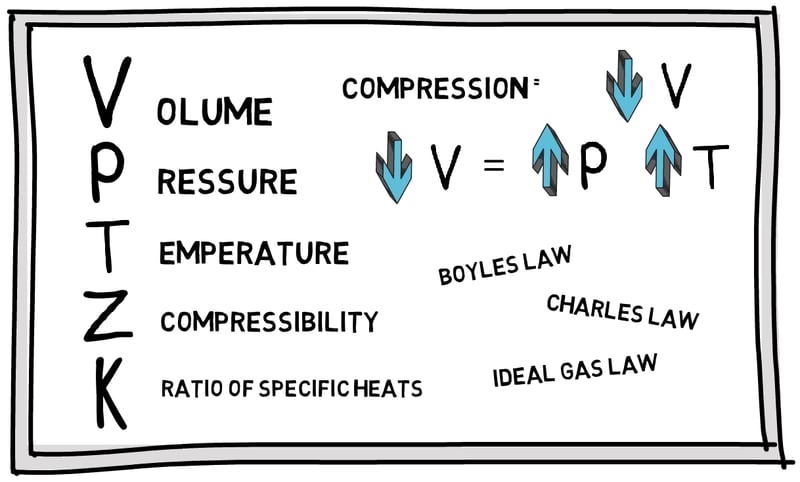
The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Learn more about this in this article.

Boyle's law, Definition, Equation, & Facts

Compressibility factor for methane.

Comparison between air and hydrogen compression gases, showing the

Everything you need to know about the wild world of heat pumps
Does applying pressure to a gas increase its temperature? - Quora
What happens when a gas is compressed? Doesn't it change into liquid as the molecules get together. Yes or no? - Quora

Spray cooling technique in liquid piston gas compression and impact of air dissolution on efficiency evaluation at different pressure levels - ScienceDirect
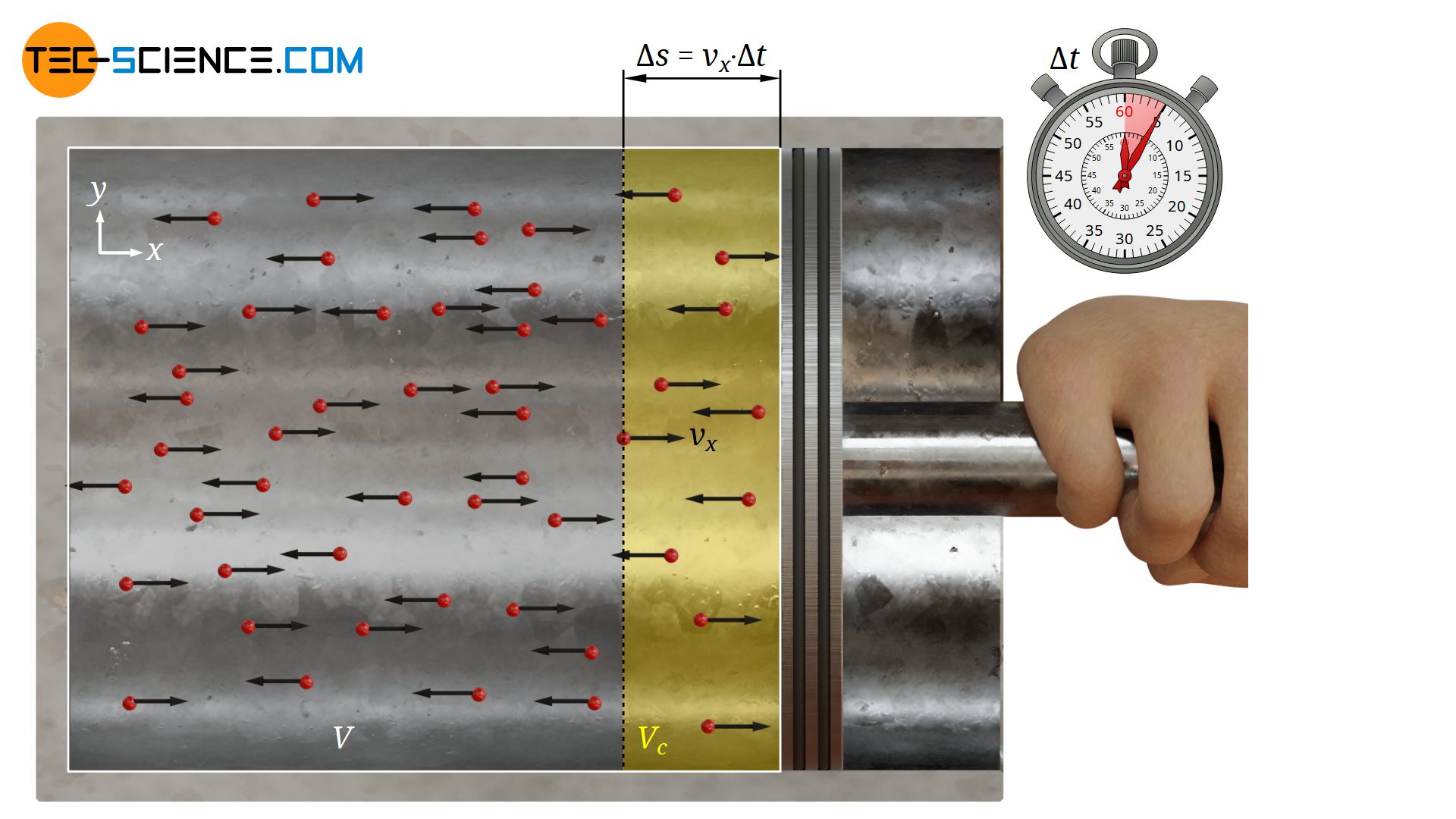
Pressure and temperature (kinetic theory of gases) - tec-science
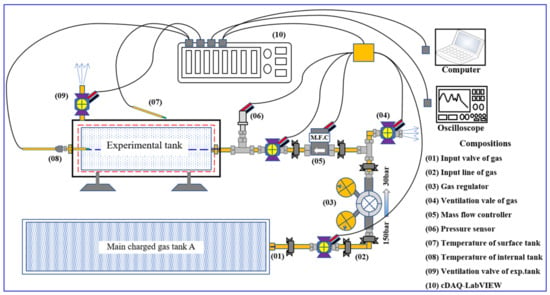
Applied Sciences, Free Full-Text

Upgradation of methane in the biogas by hydrogenation of CO2 in a prototype reactor with double pass operation over optimized Ni-Ce/Al-MCM-41 catalyst
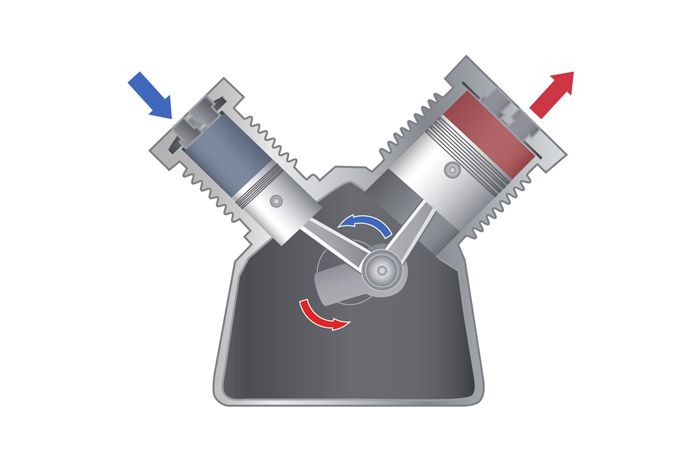
What is the first law of thermodynamics?

Top 10 Hydrogen Trends in 2024
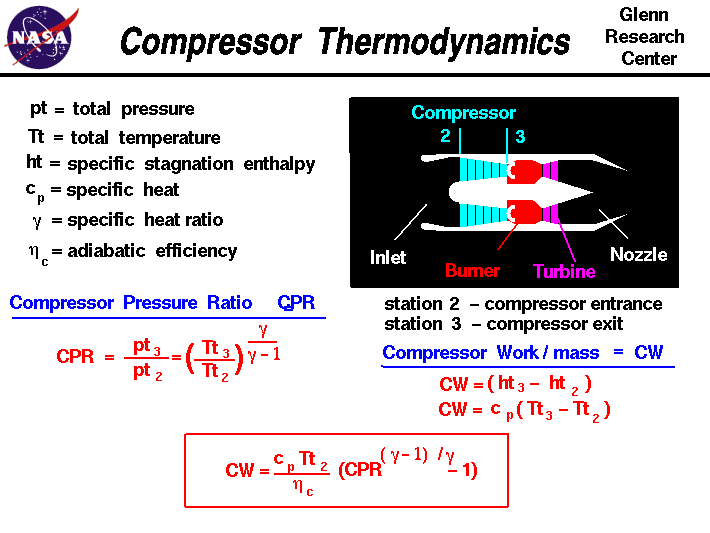
Compressor Thermodynamics